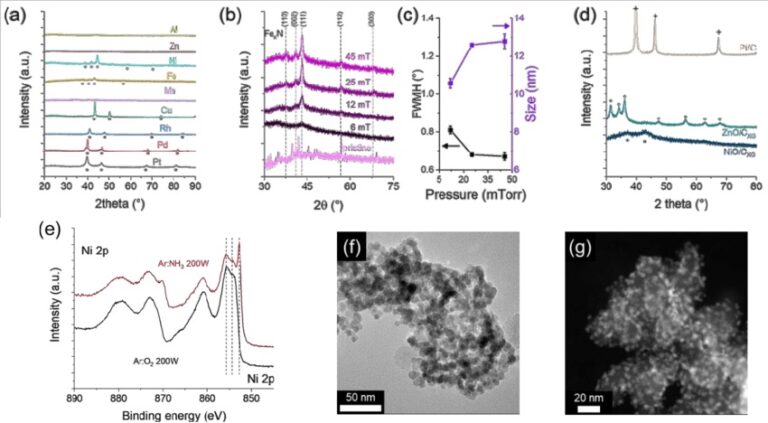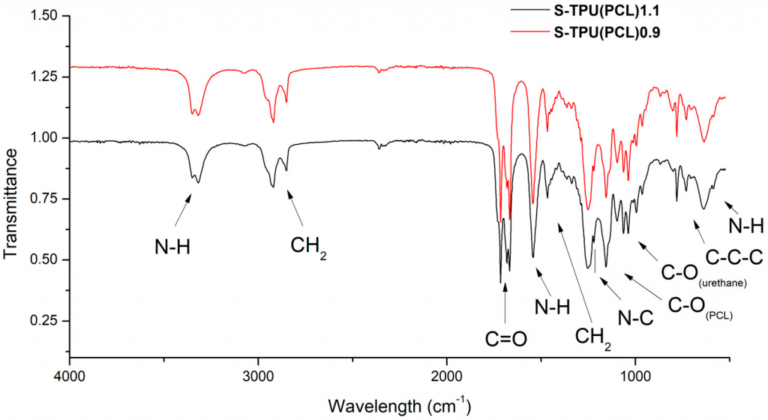
FTIR Analysis
Our scientists interpret your data and lab results
Analyst
We help researchers and students involved in interdisciplinary research and struggling with interpreting some parts of research too far out of their expertise. In order to free up your time to work on your primary research, our team of specialists assists you with project consultation and data and lab result interpretation.
FTIR Analysis: What Is It?
The most common type of infrared spectroscopy is FTIR (Fourier transform infrared) analysis. All infrared spectroscopies work on the principle that some infrared (IR) radiation is absorbed when it passes through a sample. First and foremost, it does not destroy the sample. Second, it is much faster than older techniques. Third, it is significantly more sensitive and precise.
How does FTIR analysis work?
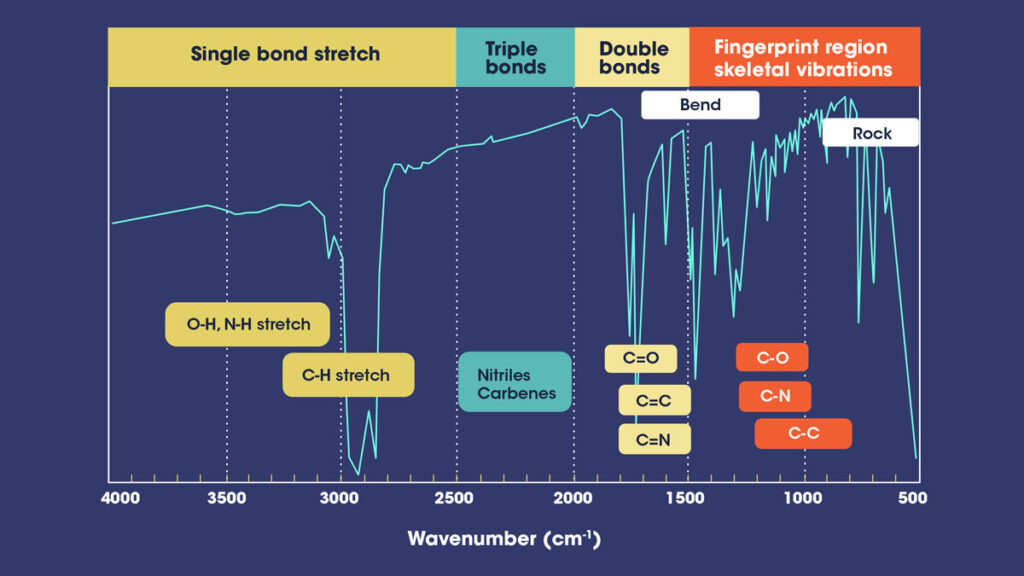
The FTIR instrument passes infrared radiation ranging from 10,000 to 100 cm-1 through a sample, with some absorbed and some passing through. The sample molecules convert the absorbed radiation into rotational and/or vibrational energy. The resulting signal at the detector appears as a spectrum, typically ranging from 4000 cm-1 to 400cm-1, representing the sample’s molecular fingerprint. Because each molecule or chemical structure produces a distinct spectral fingerprint, FTIR analysis is an excellent tool for chemical identification.
HOW TO INTERPRET FTIR SPECTRUM?
The absorbed bands in the spectrum are only marginally discrete and degenerative. The specific “peak” of energy at a given wavenumber can shift due to other chemical and matrix factors (as well as by the way the incident energy is introduced). As a result, we don’t simply have a “look up” table that tells us where a specific band of energy belongs. The spectrum must be interpreted as a whole system, requiring the most experienced analysts in all spectrographic techniques to accurately characterize the functionality presented.
Although FTIR analysis is typically used to identify materials, it can also be used to quantify specific functional groups when the chemistry is understood and standard reference materials are available. The absorbance intensity will be proportional to the amount of functionality present in the sample.
It is necessary to determine which groups and bonds correspond to which peaks when reading the spectrum. Here is a simple reference tables for the various groups.
| Frequency Range | Absorption (cm-1) | Appearance | Group | Compound Class | Comments |
|---|---|---|---|---|---|
| 4000-3000 cm-1 | 3700-3584 | medium, sharp | O-H stretching | alcohol | free |
| 3550-3200 | strong, broad | O-H stretching | alcohol | intermolecular bonded | |
| 3500 | medium | N-H stretching | primary amine | ||
| 3400 | |||||
| 3400-3300 | medium | N-H stretching | aliphatic primary amine | ||
| 3330-3250 | |||||
| 3350-3310 | medium | N-H stretching | secondary amine | ||
| 3300-2500 | strong, broad | O-H stretching | carboxylic acid | usually centered on 3000 cm-1 | |
| 3200-2700 | weak, broad | O-H stretching | alcohol | intramolecular bonded | |
| 3000-2800 | strong, broad | N-H stretching | amine salt | ||
| 3000-2500 cm-1 | |||||
| 3000-2500 cm-1 | 3333-3267 | strong, sharp | C-H stretching | alkyne | |
| 3100-3000 | medium | C-H stretching | alkene | ||
| 3000-2840 | medium | C-H stretching | alkane | ||
| 2830-2695 | medium | C-H stretching | aldehyde | doublet | |
| 2600-2550 | weak | S-H stretching | thiol | ||
| 2400-2000 cm-1 | |||||
| 2400-2000 cm-1 | 2349 | strong | O=C=O stretching | carbon dioxide | |
| 2275-2250 | strong, broad | N=C=O stretching | isocyanate | ||
| 2260-2222 | weak | CΞN stretching | nitrile | ||
| 2260-2190 | weak | CΞC stretching | alkyne | disubstituted | |
| 2175-2140 | strong | S-CΞN stretching | thiocyanate | ||
| 2160-2120 | strong | N=N=N stretching | azide | ||
| 2150 | C=C=O stretching | ketene | |||
| 2145-2120 | strong | N=C=N stretching | carbodiimide | ||
| 2140-2100 | weak | CΞC stretching | alkyne | monosubstituted | |
| 2140-1990 | strong | N=C=S stretching | isothiocyanate | ||
| 2000-1900 | medium | C=C=C stretching | allene | ||
| 2000 | C=C=N stretching | ketenimine | |||
| 2000-1650 cm-1 | |||||
| 2000-1650 cm-1 | 2000-1650 | weak | C-H bending | aromatic compound | overtone |
| 1870-1540 | |||||
| 1818 | strong | C=O stretching | anhydride | ||
| 1750 | |||||
| 1815-1785 | strong | C=O stretching | acid halide | ||
| 1800-1770 | strong | C=O stretching | conjugated acid halide | ||
| 1775 | strong | C=O stretching | conjugated anhydride | ||
| 1720 | |||||
| 1770-1780 | strong | C=O stretching | vinyl / phenyl ester | ||
| 1760 | strong | C=O stretching | carboxylic acid | monomer | |
| 1750-1735 | strong | C=O stretching | esters | 6-membered lactone | |
| 1750-1735 | strong | C=O stretching | δ-lactone | γ: 1770 | |
| 1745 | strong | C=O stretching | cyclopentanone | ||
| 1740-1720 | strong | C=O stretching | aldehyde | ||
| 1730-1715 | strong | C=O stretching | α,β-unsaturated ester | or formates | |
| 1725-1705 | strong | C=O stretching | aliphatic ketone | or cyclohexanone or cyclopentenone | |
| 1720-1706 | strong | C=O stretching | carboxylic acid | dimer | |
| 1710-1680 | strong | C=O stretching | conjugated acid | dimer | |
| 1710-1685 | strong | C=O stretching | conjugated aldehyde | ||
| 1690 | strong | C=O stretching | primary amide | free (associated: 1650) | |
| 1690-1640 | medium | C=N stretching | imine / oxime | ||
| 1685-1666 | strong | C=O stretching | conjugated ketone | ||
| 1680 | strong | C=O stretching | secondary amide | free (associated: 1640) | |
| 1680 | strong | C=O stretching | tertiary amide | free (associated: 1630) | |
| 1650 | strong | C=O stretching | δ-lactam | γ: 1750-1700 β: 1760-1730 | |
| 1670-1600 cm-1 | |||||
| 1670-1600 cm-1 | 1678-1668 | weak | C=C stretching | alkene | disubstituted (trans) |
| 1675-1665 | weak | C=C stretching | alkene | trisubstituted | |
| 1675-1665 | weak | C=C stretching | alkene | tetrasubstituted | |
| 1662-1626 | medium | C=C stretching | alkene | disubstituted (cis) | |
| 1658-1648 | medium | C=C stretching | alkene | vinylidene | |
| 1650-1600 | medium | C=C stretching | conjugated alkene | ||
| 1650-1580 | medium | N-H bending | amine | ||
| 1650-1566 | medium | C=C stretching | cyclic alkene | ||
| 1648-1638 | strong | C=C stretching | alkene | monosubstituted | |
| 1620-1610 | strong | C=C stretching | α,β-unsaturated ketone | ||
| 1600-1300 cm-1 | |||||
| 1600-1300 cm-1 | 1550-1500 | strong | N-O stretching | nitro compound | |
| 1372-1290 | |||||
| 1465 | medium | C-H bending | alkane | methylene group | |
| 1450 | medium | C-H bending | alkane | methyl group | |
| 1375 | |||||
| 1390-1380 | medium | C-H bending | aldehyde | ||
| 1385-1380 | medium | C-H bending | alkane | gem dimethyl | |
| 1370-1365 | |||||
| 1400-1000 cm-1 | |||||
| 1400-1000 cm-1 | 1440-1395 | medium | O-H bending | carboxylic acid | |
| 1420-1330 | medium | O-H bending | alcohol | ||
| 1415-1380 | strong | S=O stretching | sulfate | ||
| 1200-1185 | |||||
| 1410-1380 | strong | S=O stretching | sulfonyl chloride | ||
| 1204-1177 | |||||
| 1400-1000 | strong | C-F stretching | fluoro compound | ||
| 1390-1310 | medium | O-H bending | phenol | ||
| 1372-1335 | strong | S=O stretching | sulfonate | ||
| 1195-1168 | |||||
| 1370-1335 | strong | S=O stretching | sulfonamide | ||
| 1170-1155 | |||||
| 1350-1342 | strong | S=O stretching | sulfonic acid | anhydrous | |
| 1165-1150 | hydrate: 1230-1120 | ||||
| 1350-1300 | strong | S=O stretching | sulfone | ||
| 1160-1120 | |||||
| 1342-1266 | strong | C-N stretching | aromatic amine | ||
| 1310-1250 | strong | C-O stretching | aromatic ester | ||
| 1275-1200 | strong | C-O stretching | alkyl aryl ether | ||
| 1075-1020 | |||||
| 1250-1020 | medium | C-N stretching | amine | ||
| 1225-1200 | strong | C-O stretching | vinyl ether | ||
| 1075-1020 | |||||
| 1210-1163 | strong | C-O stretching | ester | ||
| 1205-1124 | strong | C-O stretching | tertiary alcohol | ||
| 1150-1085 | strong | C-O stretching | aliphatic ether | ||
| 1124-1087 | strong | C-O stretching | secondary alcohol | ||
| 1085-1050 | strong | C-O stretching | primary alcohol | ||
| 1070-1030 | strong | S=O stretching | sulfoxide | ||
| 1050-1040 | strong, broad | CO-O-CO stretching | anhydride | ||
| 1000-650 cm-1 | |||||
| 1000-650 cm-1 | 995-985 | strong | C=C bending | alkene | monosubstituted |
| 915-905 | |||||
| 980-960 | strong | C=C bending | alkene | disubstituted (trans) | |
| 895-885 | strong | C=C bending | alkene | vinylidene | |
| 850-550 | strong | C-Cl stretching | halo compound | ||
| 840-790 | medium | C=C bending | alkene | trisubstituted | |
| 730-665 | strong | C=C bending | alkene | disubstituted (cis) | |
| 690-515 | strong | C-Br stretching | halo compound | ||
| 600-500 | strong | C-I stretching | halo compound | ||
| 900-700 cm-1 | |||||
| 900-700 cm-1 | 880 ± 20 | strong | C-H bending | 1,2,4-trisubstituted | |
| 810 ± 20 | |||||
| 880 ± 20 | strong | C-H bending | 1,3-disubstituted | ||
| 780 ± 20 | |||||
| (700 ± 20) | |||||
| 810 ± 20 | strong | C-H bending | 1,4-disubstituted or | ||
| 1,2,3,4-tetrasubstituted | |||||
| 780 ± 20 | strong | C-H bending | 1,2,3-trisubstituted | ||
| (700 ± 20) | |||||
| 755 ± 20 | strong | C-H bending | 1,2-disubstituted | ||
| 750 ± 20 | strong | C-H bending | monosubstituted | ||
| 700 ± 20 | benzene derivative |
We work on your data to interpret and extract useful information!
Table 2.
| Compound Class | Group | Absorption (cm-1) | Appearance | Comments |
|---|---|---|---|---|
| acid halide | C=O stretching | 1815-1785 | strong | |
| alcohols | O-H stretching | 3700-3584 | medium, sharp | free |
| O-H stretching | 3550-3200 | strong, broad | intermolecular bonded | |
| O-H stretching | 3200-2700 | weak, broad | intramolecular bonded | |
| O-H bending | 1420-1330 | medium | ||
| aldehyde | C-H stretching | 2830-2695 | medium | doublet |
| C=O stretching | 1740-1720 | strong | ||
| C-H bending | 1390-1380 | medium | ||
| aliphatic ether | C-O stretching | 1150-1085 | strong | |
| aliphatic ketone | C=O stretching | 1725-1705 | strong | or cyclohexanone or cyclopentenone |
| aliphatic primary amine | N-H stretching | 3400-3300 | medium | |
| alkane | C-H stretching | 3000-2840 | medium | |
| C-H bending | 1465 | medium | methylene group | |
| C-H bending | 1450 | medium | methyl group | |
| C-H bending | 1385-1380 | medium | gem dimethyl | |
| C-H stretching | 3100-3000 | medium | ||
| C=C stretching | 1678-1668 | weak | disubstituted (trans) | |
| C=C stretching | 1675-1665 | weak | trisubstituted | |
| C=C stretching | 1675-1665 | weak | tetrasubstituted | |
| C=C stretching | 1662-1626 | medium | disubstituted (cis) | |
| C=C stretching | 1658-1648 | medium | vinylidene | |
| C=C stretching | 1648-1638 | strong | monosubstituted | |
| C=C bending | 995-985 | strong | monosubstituted | |
| C=C bending | 980-960 | strong | disubstituted (trans) | |
| C=C bending | 895-885 | strong | vinylidene | |
| C=C bending | 840-790 | medium | trisubstituted | |
| C=C bending | 730-665 | strong | disubstituted (cis) | |
| alkyl aryl ether | C-O stretching | 1275-1200 | strong | |
| alkyne | C-H stretching | 3333-3267 | strong, sharp | |
| CΞC stretching | 2260-2190 | weak | disubstituted | |
| CΞC stretching | 2140-2100 | weak | monosubstituted | |
| allene | C=C=C stretching | 2000-1900 | medium | |
| amine | N-H bending | 1650-1580 | medium | |
| C-N stretching | 1250-1020 | medium | ||
| amine salt | N-H stretching | 3000-2800 | strong, broad | |
| anhydride | C=O stretching | 1818 | strong | |
| CO-O-CO stretching | 1050-1040 | strong, broad | ||
| aromatic amine | C-N stretching | 1342-1266 | strong | |
| aromatic compound | C-H bending | 2000-1650 | weak | overtone |
| aromatic ester | C-O stretching | 1310-1250 | strong | |
| azide | N=N=N stretching | 2160-2120 | strong | |
| benzene derivative | 700 ± 20 | |||
| carbodiimide | N=C=N stretching | 2145-2120 | strong | |
| carbon dioxide | O=C=O stretching | 2349 | strong | |
| carboxylic acid | O-H stretching | 3300-2500 | strong, broad | usually centered on 3000 cm-1 |
| C=O stretching | 1760 | strong | monomer | |
| C=O stretching | 1720-1706 | strong | dimer | |
| O-H bending | 1440-1395 | medium | ||
| conjugated acid | C=O stretching | 1710-1680 | strong | dimer |
| conjugated acid halide | C=O stretching | 1800-1770 | strong | |
| conjugated aldehyde | C=O stretching | 1710-1685 | strong | |
| conjugated alkene | C=C stretching | 1650-1600 | medium | |
| conjugated anhydride | C=O stretching | 1775 | strong | |
| conjugated ketone | C=O stretching | 1685-1666 | strong | |
| cyclic alkene | C=C stretching | 1650-1566 | medium | |
| cyclopentanone | C=O stretching | 1745 | strong | |
| ester | C-O stretching | 1210-1163 | strong | |
| esters | C=O stretching | 1750-1735 | strong | 6-membered lactone |
| fluoro compound | C-F stretching | 1400-1000 | strong | |
| halo compound | C-Cl stretching | 850-550 | strong | |
| C-Br stretching | 690-515 | strong | ||
| C-I stretching | 600-500 | strong | ||
| imine / oxime | C=N stretching | 1690-1640 | medium | |
| isocyanate | N=C=O stretching | 2275-2250 | strong, broad | |
| isothiocyanate | N=C=S stretching | 2140-1990 | strong | |
| ketene | C=C=O stretching | 2150 | ||
| ketenimine | C=C=N stretching | 2000 | ||
| monosubstituted | C-H bending | 750 ± 20 | strong | |
| nitrile | CΞN stretching | 2260-2222 | weak | |
| nitro compound | N-O stretching | 1550-1500 | strong | |
| none | 3330-3250 | |||
| none | 1870-1540 | |||
| none | 1750 | |||
| none | 1720 | |||
| none | 1372-1290 | |||
| none | 1375 | |||
| none | 1370-1365 | |||
| none | 1200-1185 | |||
| none | 1204-1177 | |||
| none | 1195-1168 | |||
| none | 1170-1155 | |||
| none | 1165-1150 | hydrate: 1230-1120 | ||
| none | 1160-1120 | |||
| none | 1075-1020 | |||
| none | 1075-1020 | |||
| none | 915-905 | |||
| none | 810 ± 20 | |||
| none | 780 ± 20 | |||
| none | (700 ± 20) | |||
| none | (700 ± 20) | |||
| phenol | O-H bending | 1390-1310 | medium | |
| primary alcohol | C-O stretching | 1085-1050 | strong | |
| primary amide | C=O stretching | 1690 | strong | free (associated: 1650) |
| N-H stretching | 3500 | medium | ||
| secondary alcohol | C-O stretching | 1124-1087 | strong | |
| secondary amide | C=O stretching | 1680 | strong | free (associated: 1640) |
| secondary amine | N-H stretching | 3350-3310 | medium | |
| sulfate | S=O stretching | 1415-1380 | strong | |
| sulfonamide | S=O stretching | 1370-1335 | strong | |
| sulfonate | S=O stretching | 1372-1335 | strong | |
| sulfone | S=O stretching | 1350-1300 | strong | |
| sulfonic acid | S=O stretching | 1350-1342 | strong | anhydrous |
| sulfonyl chloride | S=O stretching | 1410-1380 | strong | |
| sulfoxide | S=O stretching | 1070-1030 | strong | |
| tertiary alcohol | C-O stretching | 1205-1124 | strong | |
| tertiary amide | C=O stretching | 1680 | strong | free (associated: 1630) |
| thiocyanate | S-CΞN stretching | 2175-2140 | strong | |
| thiol | S-H stretching | 2600-2550 | weak | |
| vinyl / phenyl ester | C=O stretching | 1770-1780 | strong | |
| vinyl ether | C-O stretching | 1225-1200 | strong | |
| α,β-unsaturated ester | C=O stretching | 1730-1715 | strong | or formates |
| α,β-unsaturated ketone | C=C stretching | 1620-1610 | strong | |
| δ-lactam | C=O stretching | 1650 | strong | γ: 1750-1700 β: 1760-1730 |
| δ-lactone | C=O stretching | 1750-1735 | strong | γ: 1770 |
| 1,2,3,4-tetrasubstituted | ||||
| 1,2,3-trisubstituted | C-H bending | 780 ± 20 | strong | |
| C-H bending | 880 ± 20 | strong | ||
| 1,2-disubstituted | C-H bending | 755 ± 20 | strong | |
| C-H bending | 880 ± 20 | strong | ||
| 1,4-disubstituted or | C-H bending | 810 ± 20 | strong |
What does FTIR serve as a tool for?
Unknown materials (e.g., films, solids, powders, or liquids) and contamination on or in a material (e.g., particles, fibers, powders) can be identified and characterized by FTIR analysis.
FTIT sample preparation:
The ability to introduce and observe energy from a specific matrix is required for proper FTIR analysis. To properly analyse the sample, we have many sample preparation and introduction techniques available in the laboratory. Transmission was the only available method of analysis in the early days of infrared spectroscopy. For transmission analysis, the sample had to be made translucent to laser and infrared energy by directly inserting it into the optical path, casting a thin film on a salt crystal, or mixing a powder version of the sample with a salt and casting.
Today, however, we can use not only transmission techniques but also reflectance techniques. We generally rely on variations of ATR (Attenuated Total Reflectance) techniques to introduce and observe energy because we can focus and manipulate the incident beam with optics. ATR involves the use of an internal reflectance phenomenon to propagate incident energy.
The beam is introduced into a crystal at an incident angle, allowing internal reflectance “bounces” at the bottom and top of the crystal before leaving on the opposite side. The sample is brought into contact with the crystal at the top, causing energy interaction at the crystal and sample interface, which is where the bounce positions are located. The more bounce positions there are, the greater the energy transfer (and thus the better the spectral response), but single bounce systems are used when a very small area needs to be analyzed.
A HATR (Horizontal Attenuated Total Reflectance) will be typically used for liquid and paste samples, which will involve placing the sample on a crystal plate or trough in a horizontal position so that gravity acts to make intimate contact with the cell. The depth of penetration into the sample can be varied by using different crystals. For example, we will use a germanium crystal for rubber analysis to limit the effect of highly IR absorbing materials in rubber (specifically carbon black), but the zinc selenide crystal is the crystal of choice for durability, moisture resistance, and penetration depth in normal everyday samples.
What are Advantages and Disadvantages of FTIR analysis?
Advantages:
- Identify compounds as small as 10 to 20 microns
- In many cases, the FTIR analysis does not harm the sample and does not alter it.
- FTIR analysis measures absorbance bands across the mid-Infrared spectrum simultaneously, allowing for a large amount of analytical information to be obtained quickly.
- Because wavelength measurement is absolute, analytical techniques such as spectral subtraction are quick and highly accurate.
Disadvantages:
- Because FTIR analysis exposes the sample to all mid-infrared frequencies at the same time, noise in one part of the radiation from the infrared source will spread throughout the spectrum.
- FTIR analysis can be affected by changes in atmospheric conditions, making it difficult to use on highly sensitive samples or samples that must be studied over a long period of time.
- Because FTIR is a bulk analysis method, it is best suited to locating and identifying broad categories of substances in a compound; however, it is less capable of identifying trace amounts of materials in mixtures with other materials.
Different types of FTIR Analysis
Transmission FTIR: In this method, the sample is placed between two transparent windows, and the infrared light passes through the sample. The amount of light absorbed by the sample at each frequency is measured, and this data is used to construct a spectrum of the sample’s chemical composition. This method is useful for analyzing samples that are transparent to infrared radiation.
Attenuated Total Reflection (ATR) FTIR: In this method, the sample is placed in contact with a crystal surface, and the infrared light is absorbed by the sample at the surface. The amount of light absorbed by the sample at each frequency is measured, and this data is used to construct a spectrum of the sample’s chemical composition. This method is useful for analyzing samples that are not transparent to infrared radiation, such as liquids or solids.
Diffuse Reflectance FTIR: In this method, the sample is ground into a powder and placed on a reflective surface, and the infrared light is reflected off the sample. The amount of light absorbed by the sample at each frequency is measured, and this data is used to construct a spectrum of the sample’s chemical composition. This method is useful for analyzing samples that are difficult to prepare in a thin, transparent film, such as powders or rough surfaces.
Fourier Transform Raman Spectroscopy (FT-Raman): In this method, the sample is irradiated with a laser, and the scattered light is analyzed by a Fourier transform spectrometer. The frequency of the scattered light is shifted due to interactions with the sample’s chemical bonds, and this shift is used to construct a spectrum of the sample’s chemical composition. This method is useful for analyzing samples that are not easily analyzed by FTIR, such as inorganic materials or samples with strong fluorescence.
Infrared Microscopy: In this method, a microscope is used to focus the infrared beam on a small area of the sample, allowing for spatially resolved measurements. The amount of light absorbed by the sample at each frequency is measured, and this data is used to construct a spectrum of the sample’s chemical composition. This method is useful for analyzing samples that have spatially varying chemical composition, such as polymer blends or biological tissues.
strengths and limitations of fifferent types of FTIR
Each type of FTIR analysis has its own strengths and limitations, and the choice of which method to use depends on the specific sample and analysis requirements.
Transmission FTIR is a commonly used method and is suitable for analyzing samples that are transparent to infrared radiation. It is also a relatively simple and straightforward method, and the data can be easily compared to reference spectra in a library.
ATR-FTIR is useful for analyzing samples that are not transparent to infrared radiation, and it can be used with a wide range of sample types, including liquids, solids, and powders. ATR-FTIR is also relatively easy to use, and the data can be compared to reference spectra in a library.
Diffuse Reflectance FTIR is useful for analyzing samples that are difficult to prepare in a thin, transparent film, such as powders or rough surfaces. It is also a relatively simple method and can be used to analyze a wide range of sample types.
FT-Raman spectroscopy is useful for analyzing samples that are not easily analyzed by FTIR, such as inorganic materials or samples with strong fluorescence. It can also be used to provide complementary information to FTIR analysis.
Infrared microscopy is useful for analyzing samples that have spatially varying chemical composition, such as polymer blends or biological tissues. It provides spatially resolved measurements and can be used to analyze small or complex samples.
In summary, the choice of which type of FTIR analysis to use depends on the specific sample and analysis requirements. Each method has its own advantages and limitations, and the most suitable method should be selected based on the nature of the sample and the analysis needs.
Is there any free FTIR database?
There are several free online databases of IR spectra, including:
- NIST Chemistry WebBook
- SDBS (Spectral Database for Organic Compounds)
- Sigma-Aldrich IR Spectra Library
- Spectral Database for Inorganic Compounds (SDBS)
- Organic Compounds Database (ThermoFisher Scientific)
- AIST Spectral Database for Organic Compounds (SDBS)
These databases can be useful for identifying unknown compounds and comparing spectra.
Learn more about step-by-step analysis of FTIR results here.
Limited offer: Get your FTIR data analyzed for just $14!
We provide interpretation for raw data obtained from the following analytical methods:
Fourier-transform infrared spectroscopy (FTIR)
Raman Spectroscopy
Inductively coupled plasma (ICP)
UV–visible (UV-Vis) spectrophotometry
X-ray powder diffraction (XRD)
Thermal gravimetric analysis (TGA)
Vibrating-sample magnetometer (VSM)
Electrochemical Impedance Spectroscopy (EIS)
Scanning Electron Microscopy (SEM)
Transmission electron microscopy (TEM)
Energy Dispersive X-ray Spectrometry (EDS)

Get your FTIR data analyzed!
Please fill in the form below and we will contact you shortly!
Discounts are available to qualified students and educators!